Racing to Save the Tasmanian Devil 4
Are you still with me? We've gotten to Hobart, met with the Director of the Save the Tasmanian Devil Program, and visited Maria Island. Now I'll get into the nitty-gritty about the DFTD plague.
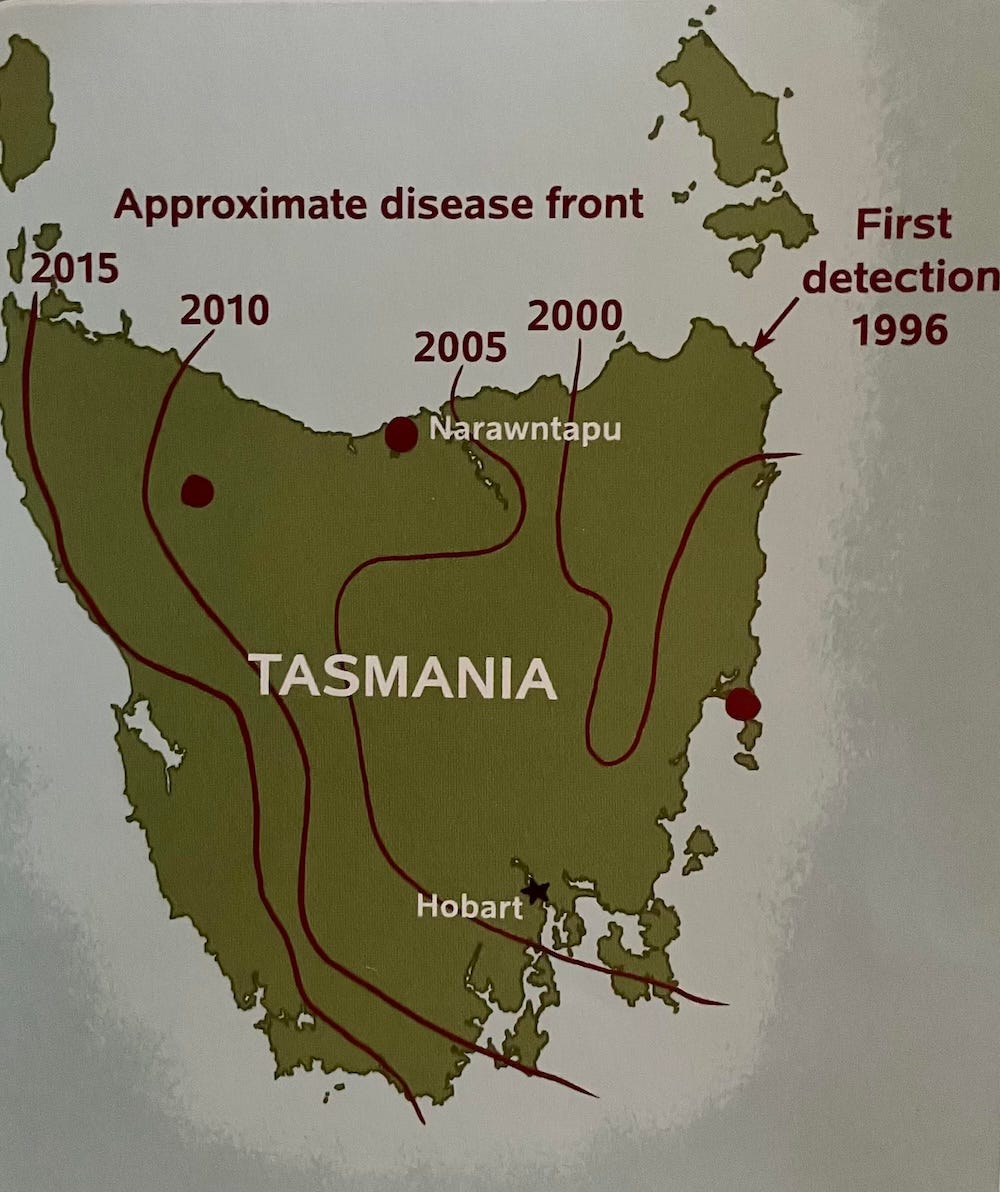
I’ve written that DFTD is a highly contagious disease that spread through Tasmania starting in 1996, killing 90% or more of the devil population up to 2015. I also noted that it was a new kind of cancer, a contagious disease that spread from one animal to another.
Here’s a Quick Explanation for what Devil Facial Tumor Disease (DTFD) is and what it does
What does that mean? Normally, the immune system recognizes any cells that are different from those in the body and kills them. That’s why the immune system needs to be quieted before an organ transplant; otherwise the immune system of the person getting the transplant would destroy it.
So how do DTFD cells keep from being recognized by the immune system of a new victim and get destroyed? Scientists from labs in England, Denmark, and Australia have cooperated in unraveling this terrible secret. They found that the cancer cells hide themselves by masking genes coding for molecules on the cell surface which identify them as foreign. It’s as if the cancer cells are wearing an invisibility cloak that keeps the newly infected devil’s immune system from “seeing” them as foreign.
Time to visit the lab that’s studying DTFD
How do scientists in laboratories actually do their work? What tools and methods do they use to learn what they want to find out? Every kind of scientific puzzle has a path that is followed to solve it, one step at a time. I was eager to see the steps being followed around understanding DFTD.

Cancer researcher Greg Woods invited me to visit his lab at the University of Tasmania’s Menzies Institute for Medical Research to see how DFTD worked on the level of cells. Once they could understand the disease process, the scientists could think about possible ways of interfering with the process.

Greg showed me how the cell cultures were kept at a carefully controlled temperature in a warm incubator. He removed one of the dishes with a cell culture, puts it under the microscope, focuses on the culture, then asks me to take a look.

‘What do these cells look like,” he asks? It’s been a long time since I looked into a microscope, and it felt good to be peeking into the lives of cells once more.
“They look pretty evenly spaced and connected to one another,” I replied.
“Good,” he responds. These are normal connective tissue cells.”
Then he showed my another culture. These cells aren’t so closely connected, but they look quite orderly. They are what are called Schwann cells, a cell type that helps the nervous system function well. A third culture has cells that are chaotic and don’t look connected.
“This last culture is made up of DFTD cells,” Greg explains. The disease is caused by cells like this, derived from Schwann cells but with no tissue structure, just chaotic cells that keep multiplying and traveling through the victim’s body as a cancer.
From Studying Cells to Developing a Vaccine
Scientists like Greg Woods can study how the cancer cells behave in the lab so that they can figure out how to develop a vaccine that can be used to help protect devils from this disease. They also preserve tissues and make thin slices that are dyed to highlight differences between normal and diseased tissues.
When I visited the lab, the scientists were already working on developing a way to protect devils from the disease. Just now, after years of development, a vaccine similar to the type used to protect people against COVID-19 is being tested. The vaccine enters devil cells, stimulating them to produce tumor cell proteins. The devil’s immune system then “knows” that these molecules are “other.” That way, if the actual disease invades the animal, its immune system recognizes those proteins as “other” and attacks the invading cells.
Devils in the Wild
Laboratory studies are only part of the effort to learn about the Tasmanian devil. It’s also important to find out how they are doing in the wild—what environments they live in, how wild populations are reproducing, and where DFTD is taking hold.
In my next and final chapter in Racing to Save the Tasmanian Devil, we’ll join graduate student Alex Fraik and her assistant Emily Burton as they maneuver a pickup with nearly bald tires up and down remote muddy roads to survey the devils in the wild.



Very interesting, Dorothy. I was intrigued by the notion of calculating what would be a good way to get the immune system to recognize those cancer cells as "other." Am I right to think that the vaccine essentially tags the intruder in some fashion?
Jon
This information is fascinating. I hope the vaccine can be prepared & distributed in time! I'm also wondering if there are other cancers that are infectious.